While I was at the observatory on a quiet Saturday, soldering away at a defective controller for a mount, I received a message from a club colleague: “Can you please take a look at which eyepiece we can use for the L-Hires [Note: a smaller spectrograph for the student lab]?” That was actually fine with me, the test of the repaired controller consisted mostly of waiting, so I had something interesting to do. So, after I got the L-Hires to work rudimentarily on a table and saw the colorful spectrum through the eyepiece, I immediately felt the need to capture it photographically. I had a camera with me anyway, so I quickly screwed it on, focused and took a snapshot at random. AHA! Lines. Amazing. Great… The question arises: What can you actually see there now? An energy-saving light bulb came to the rescue, which is in our kitchen and illuminates the coffee machine with its warm light on dark nights. If you look through a spectrograph, energy-saving light bulbs (the ones that take a long time to light up) don’t light up evenly in all colors, but show very specific lines. This is due to the mercury in the lamp. Certain individual wavelengths (i.e. colors) can be assigned to the mercury, which are conspicuously bright in the spectrum and whose wavelengths are very well known.
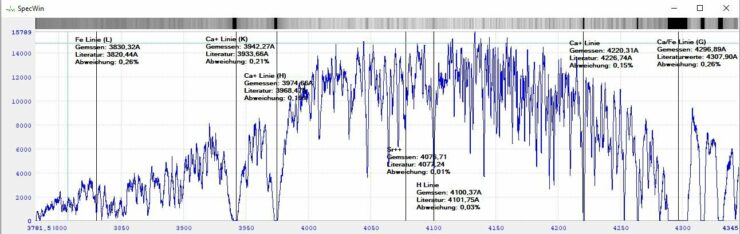
By placing the known lines from the mercury spectrum over the unknown lines from the solar spectrum, you have a clue as to where the lines are in the spectrum of the sun. The rest I found out from the literature. I was able to pick out individual prominent lines in the spectrum I had recorded and find out why this line is where it is through a highly scientific Google search. You can see the result in the picture below. You can clearly see, for example, the lines caused by the calcium in the sun, but less strongly emphasized lines such as strontium are also recognizable
All in all, a very interesting experiment that whets the appetite for more – and a good preparation for the school class coming to visit on Wednesday. This is my first shaky attempt to create and interpret a spectrum.
Photo and text: Simon Gier





Add comment